Scientific topics
Keywords
Gaëtan LIGAT Associate Professor of Virology (Maître de Conférences) - PI and Group leader
Course and current status
Associate Professor of Virology (Maître de Conférences)
Virology section member - French Society of Microbiology
Advisory board and scientific committee - Des étoiles dans la mer
Equality referent - Faculty of Sciences and Engineering & Infinity
ORCID: 0000-0003-1237-1936
Teaching activities from licence to master level
UNITEID graduate school training coordinator
Virology practical work training coordinator
Toulouse University, France
PI and Group leader: Virus and Brain Cancer
Molecular biology of host-human cytomegalovirus interactions in brain tumours and therapeutic innovations
INSERM UMR1291 - CNRS UMR5051 - Toulouse University - Infinity - Toulouse Institute for Infectious and Inflammatory Diseases, Toulouse, France
Scientific summary
Virus and Brain Cancer
Molecular biology of host-human cytomegalovirus interactions in brain tumours and therapeutic innovations
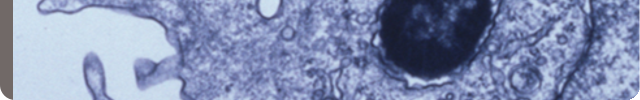
Our projects focus on the link between human cytomegalovirus (HCMV) infection and glioblastoma, an aggressive form of brain cancer.
Many of these projects have a translational aspect and include the discovery of new biomarkers and therapeutic targets.
Our lab is always looking for strong and excellent prospective Master/PhD/PostDoc candidates, so feel free to contact us if you are interested in joining the lab!
https://www.infinity.inserm.fr/en/research-teams/team-7-dunia-malnou/